Abstract
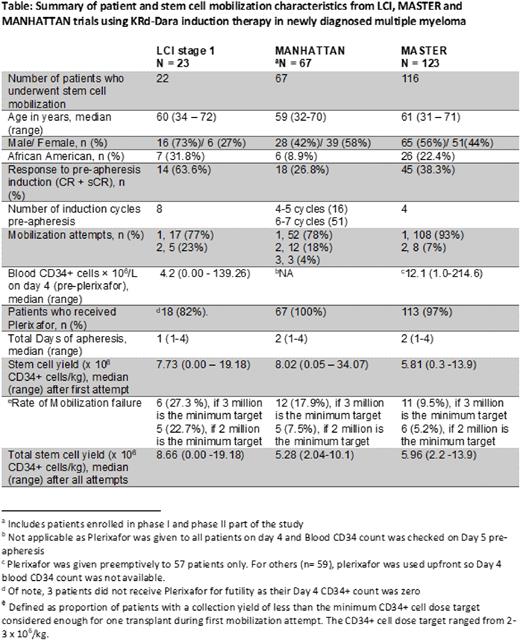
Introduction: CD38 monoclonal antibody (mAb)-containing quadruplet regimens have been shown to improve depth and duration of response in patients with NDMM eligible for autologous stem cell transplant (ASCT). However, some studies have raised concerns about the detrimental effect of combined lenalidomide and CD38 mAbs on stem cell mobilization. As an exploratory endpoint, we evaluated the effect of KRd-Dara on stem cell mobilization and collection in the Levine Cancer Institute (LCI) KRd-Dara study (NCT04113018) that permits up to 8 cycles of induction with KRd-Dara followed by an MRD-directed treatment algorithm for transplant-eligible and transplant-noneligible patients (Bhutani et al, Blood 2020 Abstr 1388). We provide a table summarizing our results from stage 1, along with two other phase II studies, the MANHATTAN (Landgren et al, JAMA Oncol 2021) and the MASTER (Costa et al, JCO 2021) studies that used variable numbers of KRd-Dara induction cycles prior to stem cell apheresis.
Patients and study design: Eligible patients had NDMM and an ECOG performance status score of 0-2. Induction treatment consisted of 28-day cycles of KRd-Dara with intravenous carfilzomib, 20/56 mg/m2 (days 1, 8, and 15); lenalidomide orally, 25 mg (days 1-21); dexamethasone weekly; daratumumab (subcutaneous) per standard approved dosing schedule. One pre-study cycle with non-protocol therapy was allowed. During stage I of the study, stem cell collection for transplant-eligible patients was performed after 8 cycles of induction.
All patients were mobilized with granulocyte-colony stimulating factor (G-CSF, 10 µg/kg) daily for 5 days. Preemptive Plerixafor was administered in the evening of Day 4 if the blood CD34+ cell count was <50/µl. Collection was done over 2 days in some patients who failed to collect sufficient CD34+ cells during the first day. A second mobilization attempt after a few weeks of additional wash-out was allowed. Our center-specific minimum required collection target is 3 x 106 CD34+ cells/kg, below which the stem cell dose is considered inadequate to perform a single ASCT. Physicians requested collection for potentially 2 or 3 transplants per patient, with a collection goal of 8 -12 x 106 CD34+ cells/kg. Mobilization failure was defined as the proportion of patients with Day 4 CD34 count <1/µl or collection of <3 x 106 CD34+ cells/kg (considered inadequate for even one ASCT) during the first mobilization attempt.
Results: Among the 22 mobilized patients during stage 1 analysis, the median age was 60 years (range, 34 to 72) and 32% were African Americans. Eighteen patients received plerixafor, 3 did not based on futility as their Day 4 blood CD34 count was zero, and only one patient met the threshold of not receiving Plerixafor. The median total CD34+ cells collected were 7.73 x 106/kg (range, 0.00-19.18). Six (27%) patients had mobilization failure after their first attempt. Four of these patients underwent a second attempt after a median of 56 days (range, 36-98 days), with only one patient collecting enough CD34+ cells for one ASCT. Of the 10 patients whose collection goal was 2 transplants (8 million CD34 cells/kg), 9 patients did not meet the goal. Of the 12 patients whose collection goal was 3 transplants (12 million CD34 cells/kg), 8 patients did not meet the goal.
Conclusions: The stem cell mobilization failure rate was high in patients collecting after 7 or 8 cycles KRd-Dara. Second remobilization with G-CSF and plerixafor was successful in only 1 of the 4 patients. We have modified stage 2 of the protocol to allow stem cell collection after cycle 3 of KRd-Dara induction. We plan to do combined analyses of LCI, MANHATTAN and MASTER to determine predictive factors associated with poor stem cell mobilization after KRd-Dara induction. Based on our results, we recommend stem cell collection after 3-4 cycles of KRd-Dara regimen.
Disclosures
Bhutani:Sanofi Genzyme: Consultancy; C4 Therapeutics: Research Funding; Avalo Therapeutics: Research Funding; Millenium: Research Funding; Bluebird bio: Research Funding; Celgene: Research Funding; Bristol Myers Squibb: Research Funding; Adaptive Biotechnologies: Research Funding; Celularity: Research Funding; Amgen: Research Funding; Legend Biotech: Research Funding; MedImmune: Research Funding; Janssen: Consultancy, Research Funding; Takeda: Research Funding. Atrash:Sanofi: Research Funding; GSK consultancy: Honoraria, Research Funding; AMGEN: Research Funding; Jansen oncology: Consultancy, Honoraria, Research Funding, Speakers Bureau; Bristol Myers Squib: Research Funding. Watts:Bristol-Myers Squibb: Consultancy. Paul:Abbvie: Membership on an entity's Board of Directors or advisory committees; Amgen: Speakers Bureau; Regeneron: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees. Chhabra:Sanofi: Research Funding; Janssen: Research Funding; GlaxoSmithKline: Honoraria; Amgen: Research Funding. Symanowski:Eli Lilly & Company: Consultancy; Immatics: Consultancy; CARsgen: Consultancy; Camurus: Consultancy. Costa:AbbVie: Research Funding; Genentech: Research Funding; Sanofi: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding. Usmani:Amgen, BMS, Janssen, Sanofi: Speakers Bureau; Abbvie, Amgen, BMS, Celgene, EdoPharma, Genentech, Gilead, GSK, Janssen,Oncopeptides, Sanofi, Seattle Genetics, SecuraBio, SkylineDX, Takeda, TeneoBio: Consultancy; Amgen, Array Biopharma, BMS, Celgene, GSK, Janssen, Merck, Pharmacyclics, Sanofi, Seattle Genetics, SkylineDX, Takeda: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.